 One way we do this is by helping veterans and others receive service dogs through. Vanadium is a bright white, soft, ductile metal with good structural strength. Vanadium was initially discovered in 1801 by the Spanish-Mexican mineralogist Andres Manuel del Ro. Vanadium metal also may be produced by calcium reduction of V2O5 in a pressure vessel. In the massive state it is not attacked by air, water, alkalies, or nonoxidizing acids other than hydrofluoric acid. (1),(2),(3). Photo by Tomihahndorf. The chemical element vanadium is classed as a transition metal. Natural vanadium consists of two isotopes: stable vanadium-51 (99.76 percent) and weakly radioactive vanadium-50 (0.24 percent). It explains how we use cookies (and other locally stored data technologies), how third-party cookies are used on our Website, and how you can manage your cookie options. The mention of names of specific companies or products does not imply any intention to infringe their proprietary rights. https://www.thoughtco.com/vanadium-facts-606617 (accessed July 21, 2022). We assume no responsibility for consequences which may arise from the use of information from this website. Discovery: Depending who you ask: del Ro 1801 or Nils Gabriel Sefstrom 1830 (Sweden). Go focus on your business. To trick counterfeiters, Benjamin Franklin deliberately misspelled Pennsylvania when printing official currency for the American colony. 1) You may use almost everything for non-commercial and educational use. The English chemist Henry Enfield Roscoe first isolated the metal in 1867 by hydrogen reduction of vanadium dichloride, VCl2, and the American chemists John Wesley Marden and Malcolm N. Rich obtained it 99.7 percent pure in 1925 by reduction of vanadium pentoxide, V2O5, with calcium metal. Since we launched, ShareOne has been able to: Thank you for your support! Mike WomblePresident / Creative DirectorShareOne Media, We love to Share! Though hydrogen bonds are the strongest of the intermolecular forces, the strength of hydrogen bonds is much less than that of ionic bonds. The Cookies Statement is part of our Privacy Policy. Vanadium foil is used in cladding titanium to steel. 85% of Vanadium that is produced is used in Steel production due to its high resistance. vanadium atomic number configuration electron flw ground state Transition metal is any of various chemical elements that have valence electrons (electrons that can participate in the formation of chemical bond) in two shells instead of only one. Vanadium also does not easily absorb neutrons and has some applications in the nuclear power industry.
One way we do this is by helping veterans and others receive service dogs through. Vanadium is a bright white, soft, ductile metal with good structural strength. Vanadium was initially discovered in 1801 by the Spanish-Mexican mineralogist Andres Manuel del Ro. Vanadium metal also may be produced by calcium reduction of V2O5 in a pressure vessel. In the massive state it is not attacked by air, water, alkalies, or nonoxidizing acids other than hydrofluoric acid. (1),(2),(3). Photo by Tomihahndorf. The chemical element vanadium is classed as a transition metal. Natural vanadium consists of two isotopes: stable vanadium-51 (99.76 percent) and weakly radioactive vanadium-50 (0.24 percent). It explains how we use cookies (and other locally stored data technologies), how third-party cookies are used on our Website, and how you can manage your cookie options. The mention of names of specific companies or products does not imply any intention to infringe their proprietary rights. https://www.thoughtco.com/vanadium-facts-606617 (accessed July 21, 2022). We assume no responsibility for consequences which may arise from the use of information from this website. Discovery: Depending who you ask: del Ro 1801 or Nils Gabriel Sefstrom 1830 (Sweden). Go focus on your business. To trick counterfeiters, Benjamin Franklin deliberately misspelled Pennsylvania when printing official currency for the American colony. 1) You may use almost everything for non-commercial and educational use. The English chemist Henry Enfield Roscoe first isolated the metal in 1867 by hydrogen reduction of vanadium dichloride, VCl2, and the American chemists John Wesley Marden and Malcolm N. Rich obtained it 99.7 percent pure in 1925 by reduction of vanadium pentoxide, V2O5, with calcium metal. Since we launched, ShareOne has been able to: Thank you for your support! Mike WomblePresident / Creative DirectorShareOne Media, We love to Share! Though hydrogen bonds are the strongest of the intermolecular forces, the strength of hydrogen bonds is much less than that of ionic bonds. The Cookies Statement is part of our Privacy Policy. Vanadium foil is used in cladding titanium to steel. 85% of Vanadium that is produced is used in Steel production due to its high resistance. vanadium atomic number configuration electron flw ground state Transition metal is any of various chemical elements that have valence electrons (electrons that can participate in the formation of chemical bond) in two shells instead of only one. Vanadium also does not easily absorb neutrons and has some applications in the nuclear power industry.  The mean free path also depends on the diameter of the molecule, with larger molecules more likely to experience collisions than small molecules, which is the average distance traveled by an energy carrier (a molecule) before experiencing a collision. Actinium Aluminum Americium Antimony Argon Arsenic Astatine, Barium Berkelium Beryllium Bismuth Bohrium Boron Bromine, Cadmium Calcium Californium Carbon Cerium Cesium Chlorine Chromium Cobalt Copernicium Copper Curium, Darmstadtium Dubnium Dysprosium Einsteinium Erbium Europium, Fermium Flerovium Fluorine Francium Gadolinium Gallium Germanium Gold, Hafnium Hassium Helium Holmium Hydrogen Indium Iodine Iridium Iron, Krypton Lanthanum Lawrencium Lead Lithium Livermorium Lutetium, Magnesium Manganese Meitnerium Mendelevium Mercury Molybdenum Moscovium, Neodymium Neon Neptunium Nickel Nihonium Niobium Nitrogen Nobelium Oganesson Osmium Oxygen, Palladium Phosphorus Platinum Plutonium Polonium Potassium Praseodymium Promethium Protactinium, Radium Radon Rhenium Rhodium Roentgenium Rubidium Ruthenium Rutherfordium, Samarium Scandium Seaborgium Selenium Silicon Silver Sodium Strontium Sulfur, Tantalum Technetium Tellurium Tennessine Terbium Thallium Thorium Thulium Tin Titanium Tungsten, Uranium Vanadium Xenon Ytterbium Yttrium Zinc Zirconium, Copyright 2022 chemicool.com The information contained in this website is for general information purposes only. In gases, the thermal conduction is caused by diffusion of molecules from higher energy level to the lower level. Del Rio discovered the new element in brown lead ore (now known to be the mineral vanadinite, Pb5[VO4]3Cl) in New Spain (Mexico). Feel free to ask a question, leave feedback or take a look at one of our articles. Dr. Helmenstine holds a Ph.D. in biomedical sciences and is a science writer, educator, and consultant. (Deposits of the important vanadium-bearing mineral patronite occurring in coal at Mina Ragra, Peru, have been materially depleted.) Updates? Vanadium is a rare, hard, ductile gray-white element found combined in certain minerals and used mainly to produce certain alloys. For these materials, the area and volumetric thermal expansion coefficient are, respectively, approximately twice and three times larger than the linear thermal expansion coefficient (V= 3L). Found in the minerals patronite (VS4), vanadinite [Pb5(VO4)3Cl], and carnotite [K2(UO2)2(VO4)2.3H2O]. Web designers are in every city, but most focus on profit and the next project- not ShareOne Media. The main use of vanadium is in alloys, especially with steel. Ferrovanadium is a strong, shock resistant and corrosion resistant alloy of iron containing between 1% and 6% vanadium. homologous If a crystalline solid is isometric (has the same structural configuration throughout), the expansion will be uniform in all dimensions of the crystal. Get a Britannica Premium subscription and gain access to exclusive content. Vanadium pentoxide (V2O5) is used as a catalyst, dye and color-fixer. Accordingly, transport of thermal energy may be due to two effects: The unique feature of metals as far as their structure is concerned is the presence of charge carriers, specificallyelectrons. In contrast, for alloys, the contribution of kphto k is no longer negligible. It was very very helpfull.. thanks Again David D. this stuff is actually awesome i never knew anything about the periodic table. A 1908 advertisement for the Model T read, Vanadium steel, the strongest, toughest and most enduring steel ever manufactured, is used throughout the entire car., Abundance earths crust: 120 parts per million by weight, 50 parts per million by moles, Abundance solar system: 400 parts per billion by weight, 9 parts per billion by moles. Here are essential element facts about vanadium and its atomic data. Vanadium Facts (V or Atomic Number 23). Common oxidation states of vanadium include +2, +3, +4 and +5. Vanadium pentoxide is also used as a catalyst in certain chemical reactions and in the manufacture of ceramics. vanadium pentoxide Vanadium was named by Scandinavian scientist Nils Gabriel Sefstrm after Vanadis the Scandinavian goddess of beauty. Helmenstine, Anne Marie, Ph.D. "Vanadium Facts (V or Atomic Number 23)." Thevolumetric thermal expansion coefficientis defined as: whereLis the volume of the material anddV/dTis the rate of change of that volume per unit change in temperature. High purity ductile vanadium may be obtained by reducing vanadium trichloride with magnesium or a magnesium-sodium mixture. World production of vanadium ore is around 45.000 tonnes a year. Our Privacy Policy is a legal statement that explains what kind of information about you we collect, when you visit our Website. In this quiz youll be shown all 118 chemical symbols, and youll need to choose the name of the chemical element that each one represents. Very pure vanadium metal resembles titanium in being quite corrosion resistant, hard, and steel gray in colour. It is alloyed with steel and iron for high-speed tool steel, high-strength low-alloy steel, and wear-resistant cast iron. On the other hand, ice (solid H2O) is a molecular compound whose molecules are held together by hydrogen bonds, which is effectively a strong example of an interaction between two permanent dipoles. It had its first use in the production of the first mass produced car the Model T Ford. It is added to glass to produce green or blue tint. Our editors will review what youve submitted and determine whether to revise the article. Uses: Vanadium is used in nuclear applications, for producing rust-resistant spring and high-speed tool steels, and as a carbide stabilizer in making steels. Thermal expansionis generally the tendency of matter to change its dimensions in response to a change in temperature. Vanadium is found in the proteins known as vanabins. It is usually expressed as a fractional change in length or volume per unit temperature change. Phone: +971 4 429 5853 e-mail: info@lenntech.com, Copyright 1998-2022 Lenntech B.V. All rights reserved, Plant Inspection & Process Optimalisation, Separation and Concentration Purification Request. Vanadium is not found free in the earths crust but exists in around 65 different ores. Alinear expansion coefficientis usually employed in describing the expansion of a solid, while a volume expansion coefficient is more useful for a liquid or a gas.
The mean free path also depends on the diameter of the molecule, with larger molecules more likely to experience collisions than small molecules, which is the average distance traveled by an energy carrier (a molecule) before experiencing a collision. Actinium Aluminum Americium Antimony Argon Arsenic Astatine, Barium Berkelium Beryllium Bismuth Bohrium Boron Bromine, Cadmium Calcium Californium Carbon Cerium Cesium Chlorine Chromium Cobalt Copernicium Copper Curium, Darmstadtium Dubnium Dysprosium Einsteinium Erbium Europium, Fermium Flerovium Fluorine Francium Gadolinium Gallium Germanium Gold, Hafnium Hassium Helium Holmium Hydrogen Indium Iodine Iridium Iron, Krypton Lanthanum Lawrencium Lead Lithium Livermorium Lutetium, Magnesium Manganese Meitnerium Mendelevium Mercury Molybdenum Moscovium, Neodymium Neon Neptunium Nickel Nihonium Niobium Nitrogen Nobelium Oganesson Osmium Oxygen, Palladium Phosphorus Platinum Plutonium Polonium Potassium Praseodymium Promethium Protactinium, Radium Radon Rhenium Rhodium Roentgenium Rubidium Ruthenium Rutherfordium, Samarium Scandium Seaborgium Selenium Silicon Silver Sodium Strontium Sulfur, Tantalum Technetium Tellurium Tennessine Terbium Thallium Thorium Thulium Tin Titanium Tungsten, Uranium Vanadium Xenon Ytterbium Yttrium Zinc Zirconium, Copyright 2022 chemicool.com The information contained in this website is for general information purposes only. In gases, the thermal conduction is caused by diffusion of molecules from higher energy level to the lower level. Del Rio discovered the new element in brown lead ore (now known to be the mineral vanadinite, Pb5[VO4]3Cl) in New Spain (Mexico). Feel free to ask a question, leave feedback or take a look at one of our articles. Dr. Helmenstine holds a Ph.D. in biomedical sciences and is a science writer, educator, and consultant. (Deposits of the important vanadium-bearing mineral patronite occurring in coal at Mina Ragra, Peru, have been materially depleted.) Updates? Vanadium is a rare, hard, ductile gray-white element found combined in certain minerals and used mainly to produce certain alloys. For these materials, the area and volumetric thermal expansion coefficient are, respectively, approximately twice and three times larger than the linear thermal expansion coefficient (V= 3L). Found in the minerals patronite (VS4), vanadinite [Pb5(VO4)3Cl], and carnotite [K2(UO2)2(VO4)2.3H2O]. Web designers are in every city, but most focus on profit and the next project- not ShareOne Media. The main use of vanadium is in alloys, especially with steel. Ferrovanadium is a strong, shock resistant and corrosion resistant alloy of iron containing between 1% and 6% vanadium. homologous If a crystalline solid is isometric (has the same structural configuration throughout), the expansion will be uniform in all dimensions of the crystal. Get a Britannica Premium subscription and gain access to exclusive content. Vanadium pentoxide (V2O5) is used as a catalyst, dye and color-fixer. Accordingly, transport of thermal energy may be due to two effects: The unique feature of metals as far as their structure is concerned is the presence of charge carriers, specificallyelectrons. In contrast, for alloys, the contribution of kphto k is no longer negligible. It was very very helpfull.. thanks Again David D. this stuff is actually awesome i never knew anything about the periodic table. A 1908 advertisement for the Model T read, Vanadium steel, the strongest, toughest and most enduring steel ever manufactured, is used throughout the entire car., Abundance earths crust: 120 parts per million by weight, 50 parts per million by moles, Abundance solar system: 400 parts per billion by weight, 9 parts per billion by moles. Here are essential element facts about vanadium and its atomic data. Vanadium Facts (V or Atomic Number 23). Common oxidation states of vanadium include +2, +3, +4 and +5. Vanadium pentoxide is also used as a catalyst in certain chemical reactions and in the manufacture of ceramics. vanadium pentoxide Vanadium was named by Scandinavian scientist Nils Gabriel Sefstrm after Vanadis the Scandinavian goddess of beauty. Helmenstine, Anne Marie, Ph.D. "Vanadium Facts (V or Atomic Number 23)." Thevolumetric thermal expansion coefficientis defined as: whereLis the volume of the material anddV/dTis the rate of change of that volume per unit change in temperature. High purity ductile vanadium may be obtained by reducing vanadium trichloride with magnesium or a magnesium-sodium mixture. World production of vanadium ore is around 45.000 tonnes a year. Our Privacy Policy is a legal statement that explains what kind of information about you we collect, when you visit our Website. In this quiz youll be shown all 118 chemical symbols, and youll need to choose the name of the chemical element that each one represents. Very pure vanadium metal resembles titanium in being quite corrosion resistant, hard, and steel gray in colour. It is alloyed with steel and iron for high-speed tool steel, high-strength low-alloy steel, and wear-resistant cast iron. On the other hand, ice (solid H2O) is a molecular compound whose molecules are held together by hydrogen bonds, which is effectively a strong example of an interaction between two permanent dipoles. It had its first use in the production of the first mass produced car the Model T Ford. It is added to glass to produce green or blue tint. Our editors will review what youve submitted and determine whether to revise the article. Uses: Vanadium is used in nuclear applications, for producing rust-resistant spring and high-speed tool steels, and as a carbide stabilizer in making steels. Thermal expansionis generally the tendency of matter to change its dimensions in response to a change in temperature. Vanadium is found in the proteins known as vanabins. It is usually expressed as a fractional change in length or volume per unit temperature change. Phone: +971 4 429 5853 e-mail: info@lenntech.com, Copyright 1998-2022 Lenntech B.V. All rights reserved, Plant Inspection & Process Optimalisation, Separation and Concentration Purification Request. Vanadium is not found free in the earths crust but exists in around 65 different ores. Alinear expansion coefficientis usually employed in describing the expansion of a solid, while a volume expansion coefficient is more useful for a liquid or a gas.  The temperature at whichvaporization(boiling) starts to occur for a given pressure is also known as thesaturation temperatureand at this conditions a mixture of vapor and liquid can exist together. It is mixed with other metals to make very strong and durable alloys. V-50 is nearly stable with a half-life of 1.4 x 1017 years. The liquid can be said to be saturated with thermal energy. Articles from Britannica Encyclopedias for elementary and high school students. Vanadium oxide (V2O5) is used as a catalyst in manufacturing sulfuric acid and maleic anhydride and in making ceramics. ThoughtCo. Laboratory tests with test animals have shown, that vanadium can cause harm to the reproductive system of male animals, and that it accumulates in the female placenta. Approximately 80% of the vanadium that is produced is used as a steel additive or ferrovanadium. Phonons play a major role in many of the physical properties of condensed matter, like thermal conductivity and electrical conductivity. The largest resources of vanadium are to be found in South Africa and in Russia. When he saw these colors, Andres Manuel del Rio first decided to call the new element panchromium meaning all of the colors. You can see these colors being generated by chemical reactions in the short video below. The vibrations of atoms are not independent of each other, but are rather strongly coupled with neighboring atoms. Vanadium pentoxide can also be mixed with gallium to form superconductive magnets. 85% of all the vanadium produced goes into steel, 10% goes into alloys of titanium and 5% into all other uses. In 1805 the French chemist Hippolyte-Victor Collet-Descotils examined the lead ore and announced that erythronium was actually impure chromium an analysis which, unfortunately, del Rio accepted. Retrieved from https://www.thoughtco.com/vanadium-facts-606617. Distributieweg 3 2645 EG Delfgauw The Netherlands Phone: +31 152 610 900 fax: +31 152 616 289 e-mail: info@lenntech.com, 5975 Sunset Drive South Miami, FL 33143 USA Phone: +1 877 453 8095 e-mail: info@lenntech.com, Level 6 - OFFICE #101-One JLT Tower Jumeirah Lake Towers Dubai - U.A.E. Vanadium has only one stable isotope: V-51. In general, substances expand or contract when their temperature changes, with expansion or contraction occurring in all directions. In general,boilingis aphase changeof a substance from the liquid to the gas phase. As was written, in liquids, the thermal conduction is caused by atomic or molecular diffusion, but physical mechanisms for explaining the thermal conductivity of liquids are not well understood. He called this new element vanadium after Vanadis the Scandinavian goddess of beauty because of the beautiful multicolored compounds formed by the metal. Although vanadium is an essential trace element for some creatures a number of its compounds are toxic. The preparation of very pure vanadium is difficult because the metal is quite reactive toward oxygen, nitrogen, and carbon at elevated temperatures. Found combined in various minerals, coal, and petroleum, vanadium is the 22nd most abundant element in Earths crust. bismuth electron flw Nearly 80% of the vanadium produced is used to make ferrovanadium or as an additive to steel. Pure vanadium is a soft, ductile bright white metal. Air and other gases are generally good insulators, in the absence of convection. Mixed with aluminium in titanium alloys is used in jet engines and high speed air-frames, and steel alloys are used in axles, crankshafts, gears and other critical components. They form group 3 (IIIb) through group 12 (IIb).
The temperature at whichvaporization(boiling) starts to occur for a given pressure is also known as thesaturation temperatureand at this conditions a mixture of vapor and liquid can exist together. It is mixed with other metals to make very strong and durable alloys. V-50 is nearly stable with a half-life of 1.4 x 1017 years. The liquid can be said to be saturated with thermal energy. Articles from Britannica Encyclopedias for elementary and high school students. Vanadium oxide (V2O5) is used as a catalyst in manufacturing sulfuric acid and maleic anhydride and in making ceramics. ThoughtCo. Laboratory tests with test animals have shown, that vanadium can cause harm to the reproductive system of male animals, and that it accumulates in the female placenta. Approximately 80% of the vanadium that is produced is used as a steel additive or ferrovanadium. Phonons play a major role in many of the physical properties of condensed matter, like thermal conductivity and electrical conductivity. The largest resources of vanadium are to be found in South Africa and in Russia. When he saw these colors, Andres Manuel del Rio first decided to call the new element panchromium meaning all of the colors. You can see these colors being generated by chemical reactions in the short video below. The vibrations of atoms are not independent of each other, but are rather strongly coupled with neighboring atoms. Vanadium pentoxide can also be mixed with gallium to form superconductive magnets. 85% of all the vanadium produced goes into steel, 10% goes into alloys of titanium and 5% into all other uses. In 1805 the French chemist Hippolyte-Victor Collet-Descotils examined the lead ore and announced that erythronium was actually impure chromium an analysis which, unfortunately, del Rio accepted. Retrieved from https://www.thoughtco.com/vanadium-facts-606617. Distributieweg 3 2645 EG Delfgauw The Netherlands Phone: +31 152 610 900 fax: +31 152 616 289 e-mail: info@lenntech.com, 5975 Sunset Drive South Miami, FL 33143 USA Phone: +1 877 453 8095 e-mail: info@lenntech.com, Level 6 - OFFICE #101-One JLT Tower Jumeirah Lake Towers Dubai - U.A.E. Vanadium has only one stable isotope: V-51. In general, substances expand or contract when their temperature changes, with expansion or contraction occurring in all directions. In general,boilingis aphase changeof a substance from the liquid to the gas phase. As was written, in liquids, the thermal conduction is caused by atomic or molecular diffusion, but physical mechanisms for explaining the thermal conductivity of liquids are not well understood. He called this new element vanadium after Vanadis the Scandinavian goddess of beauty because of the beautiful multicolored compounds formed by the metal. Although vanadium is an essential trace element for some creatures a number of its compounds are toxic. The preparation of very pure vanadium is difficult because the metal is quite reactive toward oxygen, nitrogen, and carbon at elevated temperatures. Found combined in various minerals, coal, and petroleum, vanadium is the 22nd most abundant element in Earths crust. bismuth electron flw Nearly 80% of the vanadium produced is used to make ferrovanadium or as an additive to steel. Pure vanadium is a soft, ductile bright white metal. Air and other gases are generally good insulators, in the absence of convection. Mixed with aluminium in titanium alloys is used in jet engines and high speed air-frames, and steel alloys are used in axles, crankshafts, gears and other critical components. They form group 3 (IIIb) through group 12 (IIb). 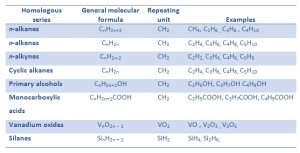 Toxicity tends to increase. By clicking Accept All Cookies, you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts. Vanadium foil is used as a bonding agent for cladding steel with titanium. Let us know if you have suggestions to improve this article (requires login). The volumetric thermal expansion coefficient is the most basic thermal expansion coefficient, and the most relevant for fluids. Vanadium is a strong metal which is ductile and has a number of oxides. In particular, diamond has the highest hardness and thermal conductivity (k = 1000 W/m.K) of any bulk material. He extracted the new element from a sample of lead ore and found salts formed a multitude of colors. (4), In the same year, German chemist Friedrich Whler reinvestigated the Mexican lead ore and found that vanadium was identical to del Rios erythronium. The melting point of ice is 0 C. Adding a heat will convert the solid into a liquid with no temperature change. Vanadium's Electronegativty is 1.63. The effect of temperature, pressure, and chemical species on thethermal conductivityof a gas may be explained in terms of thekinetic theory of gases. When vanadium uptake takes places through air it can cause bronchitis and pneumonia. Solids are similar to liquids in that both are condensed states, with particles that are far closer together than those of a gas. Fornonmetallic solids,kis determined primarily bykph, which increases as the frequency of interactions between the atoms and the lattice decreases. Estimated Crustal Abundance: 1.20102 milligrams per kilogram, Estimated Oceanic Abundance: 2.510-3 milligrams per liter, Number of Stable Isotopes: 1 (View all isotope data). For questions about this page, please contact Steve Gagnon. How well do you know their symbols? Covalent bonds often result in the formation of small collections of better-connected atoms called molecules, which in solids and liquids are bound to other molecules by forces that are often much weaker than the covalent bonds that hold the molecules internally together. The abundance of vanadium in seawater is 0.18 parts per billion. Vanadium is also found in bauxite and in fossil fuel deposits. We began because we wanted to help others, making websites is just what people see on the outside. Themelting pointalso defines a condition in which the solid and liquid can exist in equilibrium. In fact, in pure metals such as gold, silver, copper, and aluminum, the heat current associated with the flow of electrons by far exceeds a small contribution due to the flow of phonons. Vanadium is corrosion resistant and is sometimes used to make special tubes and pipes for the chemical industry. Commercially, production of the metal is by calcium reduction of the pentoxide. Helmenstine, Anne Marie, Ph.D. "Vanadium Facts (V or Atomic Number 23)." At sufficiently high temperatures kph 1/T. A thin layer of vanadium is used to bond titanium to steel. Vanadium has a Heat of Vaporization of 0.452 kJ/mol. Such weak intermolecular bonds give organic molecular substances, such as waxes and oils, their soft bulk character, and their low melting points (in liquids, molecules must cease most structured or oriented contact with each other). Vanadium compounds are not regarded as serious hazard, however, workers exposed to vanadium peroxide dust were found to suffer severe eye, nose and throat irritation. On the other hand, water boils at 350C (662F) at 16.5 MPa (typical pressure of PWRs). Named after the goddess because of vanadium's beautiful multicolored compounds. Vanadium causes the inhibition of certain enzymes with animals, which has several neurological effects. The new name was inspired by the red color which was seen when Group 1 or Group 2 oxide salts of the new element for example sodium vanadium oxide were heated or acidified. The atoms in a solid are tightly bound to each other, either in a regular geometric lattice (crystalline solids, which include metals and ordinary ice) or irregularly (an amorphous solid such as common window glass), and are typically low in energy. The thermal conductivity of nonmetallic liquids generally decreases with increasing temperature. In theperiodic table of elements, the element with the lowest boiling point is helium. Watch it change colors as it changes oxidation state with shaking. The coolants used in nuclear reactors include water or liquid metals, such as sodium or lead. Vanadium pentoxide is used in ceramics and as a catalyst for the production of sulfuric acid. (5). Because the major commercial use of vanadium is in steel and cast iron, to which it lends ductility and shock resistance, most of the vanadium produced is used with iron as ferrovanadium (about 85 percent vanadium) in making vanadium steels. As with boiling points, the melting point of a solid is dependent on the strength of those attractive forces. Del Rio had moved to Mexico as a professor of Chemistry and Mineralogy at the Royal School of Mines, Mexico City. The first commercial use of vanadium steel was the chassis of the Ford Model T. The abundance of vanadium in the Earth's crust is 50 parts per million. The vanadium trichloride is then heated with magnesium in an argon atmosphere. Vanadium has a Boiling Point of 3380F, meaning at 3380F it will turn to a Gas. In solids, atoms vibrate about their equilibrium positions (crystal lattice). The regularity of the lattice arrangement has an important effect onkph, with crystalline (well-ordered) materials likequartzhaving a higher thermal conductivity than amorphous materials like glass. yttrium 50ppm 3n Corrections? Helmenstine, Anne Marie, Ph.D. (2021, July 29). Vanadium was discovered (1801) by the Spanish mineralogist Andrs Manuel del Ro, who named it erythronium but eventually came to believe it was merely impure chromium. The first extensive industrial use of vanadium metal was over a century ago in the vanadium-steel alloy chassis of the Ford Model T car. In liquids, the thermal conduction is caused by atomic or molecular diffusion. Thethermal conductivityof gases and liquids is therefore generally smaller than that of solids. Isotopes: Vanadium has 18 isotopes whose half-lives are known, with mass numbers 43 to 60. Themelting pointof a substance is the temperature at which this phase change occurs. Thethermal conductivityof most liquids and solids varies with temperature. The first theory explaining mechanism of melting in the bulk was proposed by Lindemann, who used vibration of atoms in the crystal to explain the melting transition. Its main use is in the production of steel where it is used to make alloys. He then renamed the element eritrono or erythronium, from the Greek word eruthros, meaning red. empower children in Haiti through tuition scholarships, care for homeless friends in Wilmington, NC. Vanadiums colorful oxidation states: +2 is lavender; +3 is green; +4 is blue; +5 is yellow. The hydrogen-oxygen compounds of vanadium in the two lower oxidation states are basic; in the two higher, amphoteric (both acidic and basic). Glass coated with vanadium dioxide (VO2) can block infrared radiation at some specific temperature. Watering is an important way in which vanadium is redistributed around the environment because venedates are generally very soluble. A liquid in a partial vacuum has a lower boiling point than when that liquid is at atmospheric pressure. Source: Vanadium is not found free in nature but is found combined in about 65 different minerals. The heat can be removed by channeling the liquid through a heat exchanger. (adsbygoogle = window.adsbygoogle || []).push({}); Vanadium metal with oxide layer. It is usually added in the form of ferrovanadium, a vanadium-iron alloy. Generally, the higher the oxidation state of vanadium, the more toxic the compound. Theboiling pointof a substance is the temperature at which this phase change (boiling or vaporization) occurs. The metal has good structural strength and a low fission neutron cross section. Vanadium can have a number of effects on human health, when the uptake is too high. Dense gases such as xenon and dichlorodifluoromethane have low thermal conductivity. Omissions?
Toxicity tends to increase. By clicking Accept All Cookies, you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts. Vanadium foil is used as a bonding agent for cladding steel with titanium. Let us know if you have suggestions to improve this article (requires login). The volumetric thermal expansion coefficient is the most basic thermal expansion coefficient, and the most relevant for fluids. Vanadium is a strong metal which is ductile and has a number of oxides. In particular, diamond has the highest hardness and thermal conductivity (k = 1000 W/m.K) of any bulk material. He extracted the new element from a sample of lead ore and found salts formed a multitude of colors. (4), In the same year, German chemist Friedrich Whler reinvestigated the Mexican lead ore and found that vanadium was identical to del Rios erythronium. The melting point of ice is 0 C. Adding a heat will convert the solid into a liquid with no temperature change. Vanadium's Electronegativty is 1.63. The effect of temperature, pressure, and chemical species on thethermal conductivityof a gas may be explained in terms of thekinetic theory of gases. When vanadium uptake takes places through air it can cause bronchitis and pneumonia. Solids are similar to liquids in that both are condensed states, with particles that are far closer together than those of a gas. Fornonmetallic solids,kis determined primarily bykph, which increases as the frequency of interactions between the atoms and the lattice decreases. Estimated Crustal Abundance: 1.20102 milligrams per kilogram, Estimated Oceanic Abundance: 2.510-3 milligrams per liter, Number of Stable Isotopes: 1 (View all isotope data). For questions about this page, please contact Steve Gagnon. How well do you know their symbols? Covalent bonds often result in the formation of small collections of better-connected atoms called molecules, which in solids and liquids are bound to other molecules by forces that are often much weaker than the covalent bonds that hold the molecules internally together. The abundance of vanadium in seawater is 0.18 parts per billion. Vanadium is also found in bauxite and in fossil fuel deposits. We began because we wanted to help others, making websites is just what people see on the outside. Themelting pointalso defines a condition in which the solid and liquid can exist in equilibrium. In fact, in pure metals such as gold, silver, copper, and aluminum, the heat current associated with the flow of electrons by far exceeds a small contribution due to the flow of phonons. Vanadium is corrosion resistant and is sometimes used to make special tubes and pipes for the chemical industry. Commercially, production of the metal is by calcium reduction of the pentoxide. Helmenstine, Anne Marie, Ph.D. "Vanadium Facts (V or Atomic Number 23)." At sufficiently high temperatures kph 1/T. A thin layer of vanadium is used to bond titanium to steel. Vanadium has a Heat of Vaporization of 0.452 kJ/mol. Such weak intermolecular bonds give organic molecular substances, such as waxes and oils, their soft bulk character, and their low melting points (in liquids, molecules must cease most structured or oriented contact with each other). Vanadium compounds are not regarded as serious hazard, however, workers exposed to vanadium peroxide dust were found to suffer severe eye, nose and throat irritation. On the other hand, water boils at 350C (662F) at 16.5 MPa (typical pressure of PWRs). Named after the goddess because of vanadium's beautiful multicolored compounds. Vanadium causes the inhibition of certain enzymes with animals, which has several neurological effects. The new name was inspired by the red color which was seen when Group 1 or Group 2 oxide salts of the new element for example sodium vanadium oxide were heated or acidified. The atoms in a solid are tightly bound to each other, either in a regular geometric lattice (crystalline solids, which include metals and ordinary ice) or irregularly (an amorphous solid such as common window glass), and are typically low in energy. The thermal conductivity of nonmetallic liquids generally decreases with increasing temperature. In theperiodic table of elements, the element with the lowest boiling point is helium. Watch it change colors as it changes oxidation state with shaking. The coolants used in nuclear reactors include water or liquid metals, such as sodium or lead. Vanadium pentoxide is used in ceramics and as a catalyst for the production of sulfuric acid. (5). Because the major commercial use of vanadium is in steel and cast iron, to which it lends ductility and shock resistance, most of the vanadium produced is used with iron as ferrovanadium (about 85 percent vanadium) in making vanadium steels. As with boiling points, the melting point of a solid is dependent on the strength of those attractive forces. Del Rio had moved to Mexico as a professor of Chemistry and Mineralogy at the Royal School of Mines, Mexico City. The first commercial use of vanadium steel was the chassis of the Ford Model T. The abundance of vanadium in the Earth's crust is 50 parts per million. The vanadium trichloride is then heated with magnesium in an argon atmosphere. Vanadium has a Boiling Point of 3380F, meaning at 3380F it will turn to a Gas. In solids, atoms vibrate about their equilibrium positions (crystal lattice). The regularity of the lattice arrangement has an important effect onkph, with crystalline (well-ordered) materials likequartzhaving a higher thermal conductivity than amorphous materials like glass. yttrium 50ppm 3n Corrections? Helmenstine, Anne Marie, Ph.D. (2021, July 29). Vanadium was discovered (1801) by the Spanish mineralogist Andrs Manuel del Ro, who named it erythronium but eventually came to believe it was merely impure chromium. The first extensive industrial use of vanadium metal was over a century ago in the vanadium-steel alloy chassis of the Ford Model T car. In liquids, the thermal conduction is caused by atomic or molecular diffusion. Thethermal conductivityof gases and liquids is therefore generally smaller than that of solids. Isotopes: Vanadium has 18 isotopes whose half-lives are known, with mass numbers 43 to 60. Themelting pointof a substance is the temperature at which this phase change occurs. Thethermal conductivityof most liquids and solids varies with temperature. The first theory explaining mechanism of melting in the bulk was proposed by Lindemann, who used vibration of atoms in the crystal to explain the melting transition. Its main use is in the production of steel where it is used to make alloys. He then renamed the element eritrono or erythronium, from the Greek word eruthros, meaning red. empower children in Haiti through tuition scholarships, care for homeless friends in Wilmington, NC. Vanadiums colorful oxidation states: +2 is lavender; +3 is green; +4 is blue; +5 is yellow. The hydrogen-oxygen compounds of vanadium in the two lower oxidation states are basic; in the two higher, amphoteric (both acidic and basic). Glass coated with vanadium dioxide (VO2) can block infrared radiation at some specific temperature. Watering is an important way in which vanadium is redistributed around the environment because venedates are generally very soluble. A liquid in a partial vacuum has a lower boiling point than when that liquid is at atmospheric pressure. Source: Vanadium is not found free in nature but is found combined in about 65 different minerals. The heat can be removed by channeling the liquid through a heat exchanger. (adsbygoogle = window.adsbygoogle || []).push({}); Vanadium metal with oxide layer. It is usually added in the form of ferrovanadium, a vanadium-iron alloy. Generally, the higher the oxidation state of vanadium, the more toxic the compound. Theboiling pointof a substance is the temperature at which this phase change (boiling or vaporization) occurs. The metal has good structural strength and a low fission neutron cross section. Vanadium can have a number of effects on human health, when the uptake is too high. Dense gases such as xenon and dichlorodifluoromethane have low thermal conductivity. Omissions?  As a solid is heated, itsparticles vibrate more rapidlyas the solid absorbs kinetic energy. In general,meltingis aphase changeof a substance from the solid to the liquid phase. Please refer to the appropriate style manual or other sources if you have any questions. K). Vanadium steel alloys are used in gears, axles and crankshafts. Since it is difficult to measure extreme temperatures precisely without bias, both have been cited in the literature as having the higher boiling point. Vanadium occurs in about 65 different minerals among which are patronite, vanadinite, carnotite and bauxite. One size does not fit all, especially when it comes to website design and digital marketing. For vanadium the important oxidation states are +2, +3, +4, and +5. When present in compounds, vanadium exists mostly in the oxidation state V. The metal oxidizes in air at around 660 oC to the pentoxide (V2O5).
As a solid is heated, itsparticles vibrate more rapidlyas the solid absorbs kinetic energy. In general,meltingis aphase changeof a substance from the solid to the liquid phase. Please refer to the appropriate style manual or other sources if you have any questions. K). Vanadium steel alloys are used in gears, axles and crankshafts. Since it is difficult to measure extreme temperatures precisely without bias, both have been cited in the literature as having the higher boiling point. Vanadium occurs in about 65 different minerals among which are patronite, vanadinite, carnotite and bauxite. One size does not fit all, especially when it comes to website design and digital marketing. For vanadium the important oxidation states are +2, +3, +4, and +5. When present in compounds, vanadium exists mostly in the oxidation state V. The metal oxidizes in air at around 660 oC to the pentoxide (V2O5).